Sample 12052
Pigeonite Basalt 1866 grams
Section titled “Pigeonite Basalt 1866 grams”
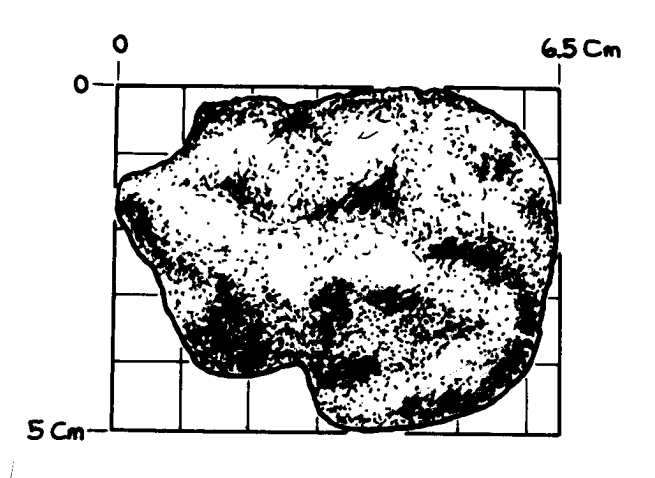
Figure 1: Photo of broken side of 12052 showing vugs. Sample is 6.5 cm high. NASA # S70-44633.
Summary of Age Data for 12052
Section titled “Summary of Age Data for 12052”Ar/Ar Rb/Sr Nyquist 1977 (recalculated)
Murthy et al. 1971 2.92 ± 0.18 b.y. Compston et al. 1971 3.28 ± 0.19 (3.22 ± 0.19)
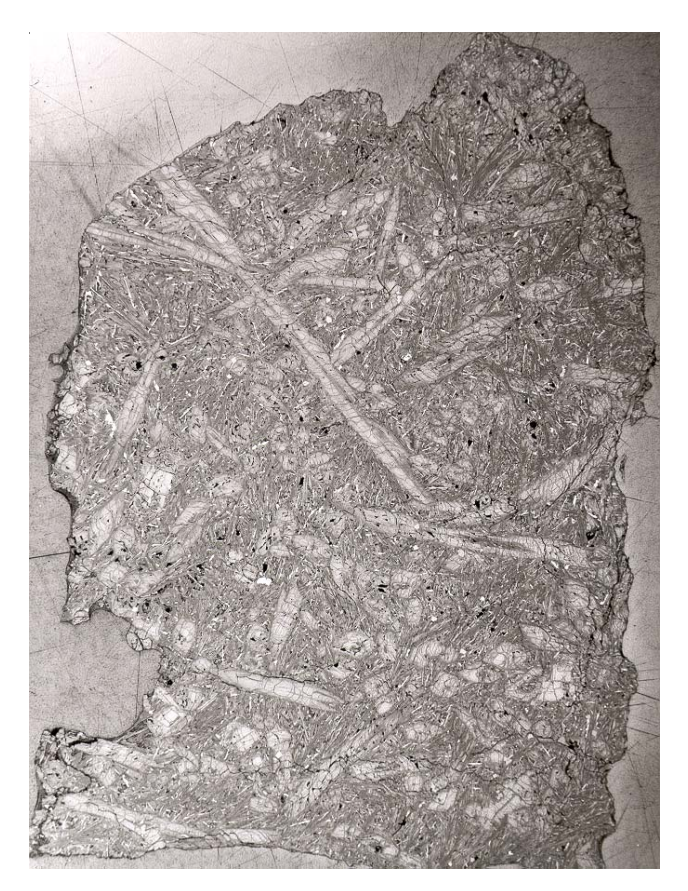
Figure 2a: Reflected light photo of thin section 12052,6. Scale about 3 cm. NASA #S70-30232.
Figure 2b: Transmitted light photo of thin section 12052,6. NASA #S70-25409.
Introduction
Section titled “Introduction”12052 is a porphyritic pigeonite basalt about 3.2 b.y old. It was rounded and covered with zap pits on all sides, before it was broken (figures 1 and 9).
Petrography
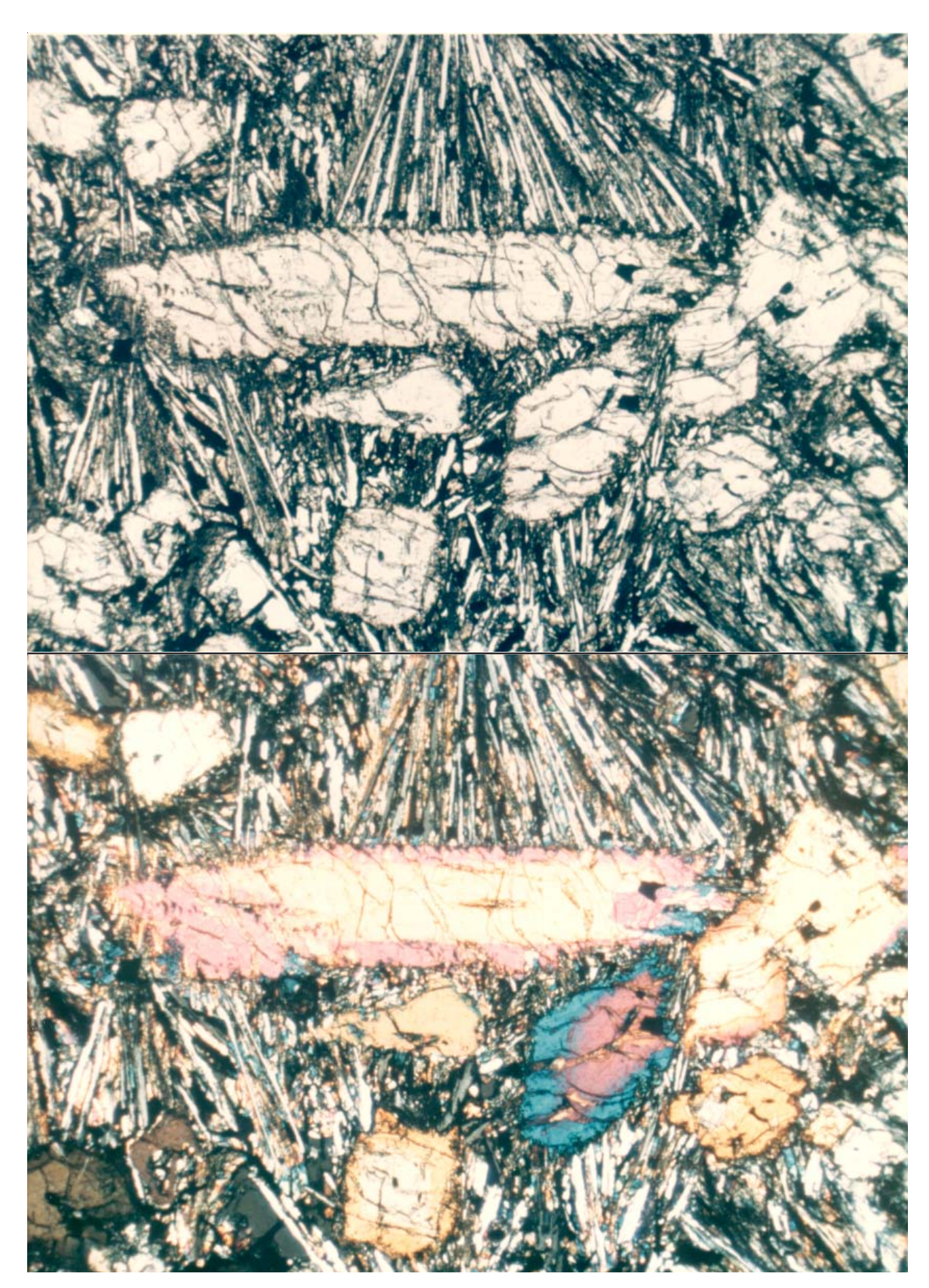
Section titled “Petrography”Bence et al. (1970) reported that 12052 is composed of “euhedral and skeletal phenocrysts of olivine and clinopyroxene (pigeonite cores with augite rims) surrounded by a groundmass including high-iron clinopyroxene, chrome spinel, ilmenite, pyroxferroite and anorthite”. Bence et al. (1971) described the rock as “porphyritic” with variolitic groundmass (figures 2
and 3). Champness et al. (1971) explain that “this rock consists of elongated phenocrysts of pyroxene (3-4 mm) in random orientation, together with skeletal or rounded olivine crystals (1 mm) in a finer-grained variolitic groundmass of pyroxene-plagioclase and opaques minerals, chiefly ilmenites, which are parallel to the pyroxene laths.”
Mineralogy
Section titled “Mineralogy”Olivine: Champness et al. (1971) report olivine is Fo71.
Mineralogical Mode for 12052
| willici alugica | 1 1410uc 101 120. | 34 | |
|---|---|---|---|
| Neal et | Papike et | Champness | |
| al. 1994 | al. 1976 | et al. 1971 | |
| Olivine | 1 | 3.9 | 5 |
| Pyroxene | 57.9 | 68.1 | |
| Plagioclase | 31.7 | 17.2 | |
| Opaques | 10.8 | 7 | |
| Ilmenite | 3.5 | ||
| Chromite +Usp | 2.5 | ||
| mesostasis | 3 | ||
| ”silica” | trace |
Pyroxene: Bence et al. (1970, 1971) studied the complex zoning patterns in the large pyroxenes in 12052. Pigeonite cores have sharp boundaries with augite rims (figure 4). Pyroxene
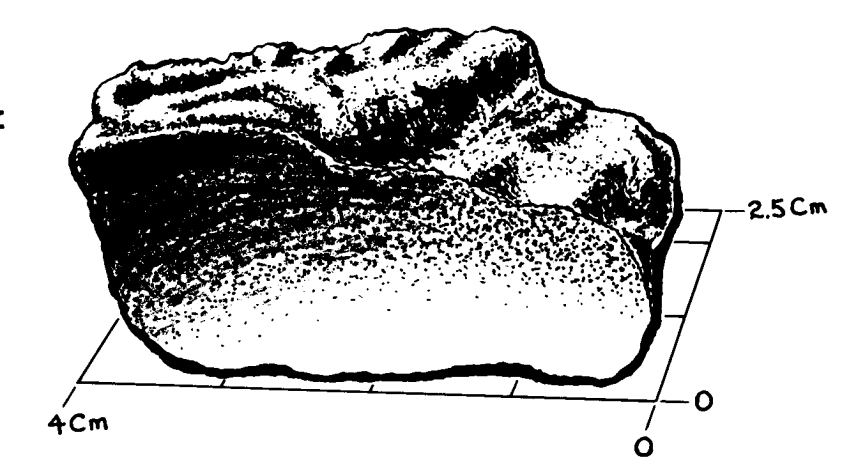
Figure 3,a,b: Photomicrographs of thin section 12052,7 showing zoned pyroxene phenocrysts in cross section and variolitic groundmass. Scale is about 2.5 mm across. NASA #S 70-49562-563.
Lunar Sample Compendium C Meyer 2009
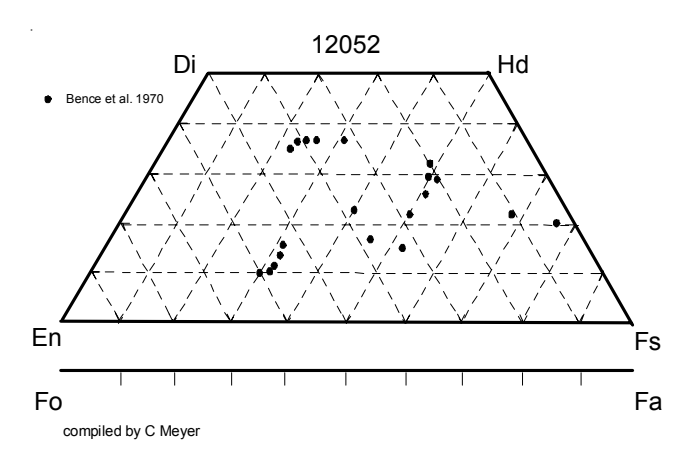
Figure 4: Bence et al. (1970, 1971) determined the composition of pyroxene in 12052.
rim and groundmass pyroxene is increasingly Fe-rich. Papike et al. (1971) and Takeda (1971) reported crystallographic data and discussed epitaxy and exsolution of pyroxenes in 12052. Champness et al. (1971) used high-voltage TEM to study fine structure (exsolution).
Plagioclase: Plagioclase crystals in 12052 are narrow (55 microns) (Baldridge et al. 1979).
Ilmenite: Ilmenite in 12052 occurs as lath-shaped crystals up to 400 micron long and 50 micron wide, typically found intergrown with plumose plagioclasepyroxene aggregates of the groundmass (Gibb et al. 1970).
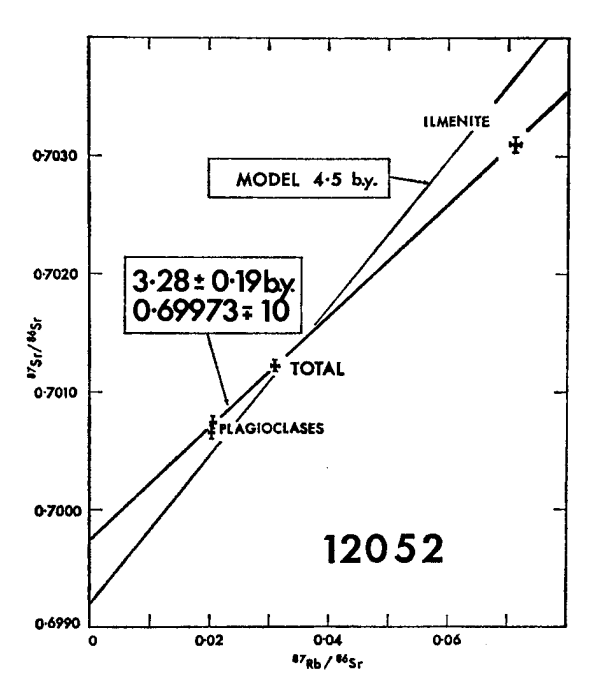
Figure 7: Rb/Sr isochron diagram for 12052 (from Compston et al. 1971).
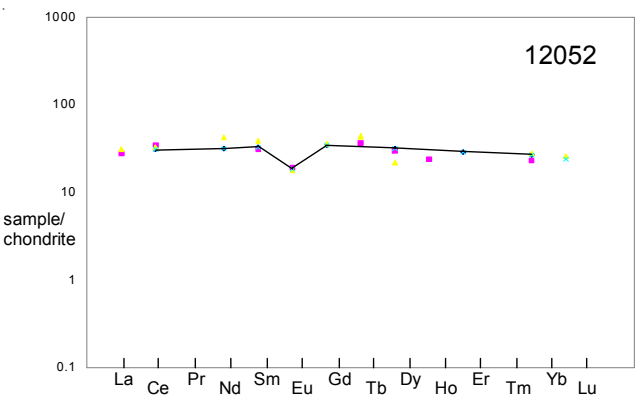
Figure 5: Normalized rare-earth-element diagram for 12052 (data from Wanke et al. connected).
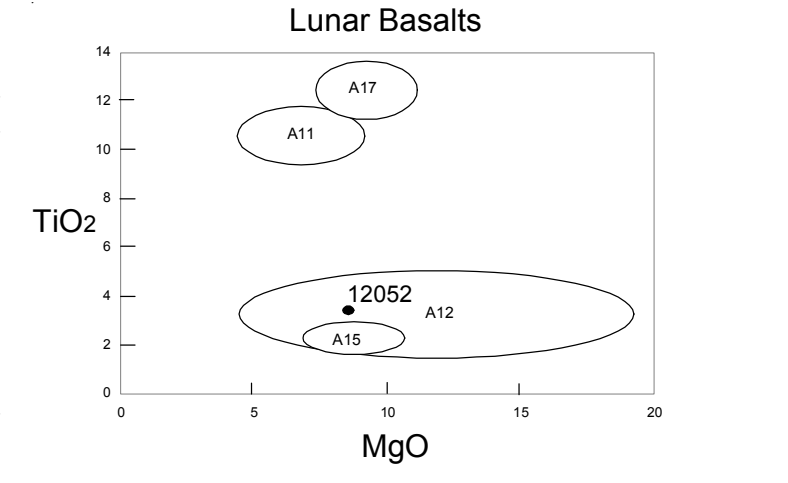
Figure 6: Composition of 12052 compared with that of other lunar basalts.
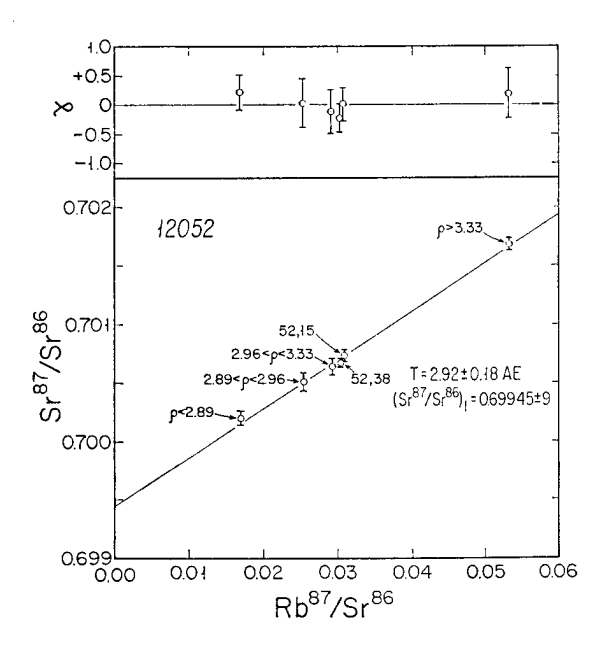
Figure 8: Rb/Sr isochron for 12052 (from Murthy et al 1971).
Table 1a. Chemical composition of 12052.
| reference Maxwell71 Kushiro71 LSPET70 | Murthy71 | O’Kelly71 | Wanke71 | Schnetzler71 Compston71 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| weight SiO2 % TiO2 Al2O3 FeO MnO MgO CaO Na2O K2O P2O5 S % sum | 46.59 3.3 10.24 19.82 0.258 8.14 10.7 0.27 0.067 0.083 | 1 g 46.49 3.18 10.29 19.93 0.27 8.44 10.82 0.28 0.07 0.03 | (f) 42 3.6 11 21 0.31 10 11 0.45 0.069 | (c ) | 0.054 | 0.058 | 201 g (d) 0.065 | 47.3 2.5 10.1 19.6 0.28 8.46 11.9 0.24 | (a) (a) (a) (a) (a) (a) (a) (a) | 204 mg (e) 0.067 (a) 0.064 | 46.13 3.35 9.95 20.7 0.28 8.07 10.89 0.26 (d) 0.071 0.08 0.07 | (g) (g) (g) (g) (g) (g) (g) (g) (g) (g) (g) | ||||||
| Sc ppm V | 52.3 160 | (b) (b) | 52 105 | 50.6 | (a) | 149 | (g) | |||||||||||
| Cr Co Ni Cu | 3675 42 23 16 | (b) (b) (b) | (b) 3763 | 3700 42 32 | 3490 38.4 39 39 | (a) (a) (a) (a) | 3140 34 6 8 | (g) (g) (g) (g) | ||||||||||
| Zn Ga Ge ppb As | 3.9 60 0.006 (a) | (a) (a) | 9 2.1 | (g) (g) | ||||||||||||||
| Se Rb Sr Y Zr Nb Mo Ru Rh Pd ppb | 85 48 150 | (b) (b) (b) | 0.8 135 42 170 | 1.12 105 | 1.26 110 | (d) (d) | 110 | 1.26 (a) 116 | (d) 1.22 (d) 113.7 40 121 7 | (g) (g) (g) | (g) 1.26 (g) 113.8 | (d) (d) | ||||||
| Ag ppb Cd ppb In ppb Sn ppb Sb ppb Te ppb Cs ppm | 7.8 | (a) | ||||||||||||||||
| Ba La Ce | 7.4 20 | (a) (a) | 50 | 66 | 69 | (d) | 70 6.52 21 | (a) | (a) 75 (a) 18.8 | (d) 70 5 (d) 12 | (g) (g) (g) | |||||||
| Pr Nd Sm Eu Gd Tb | 19.5 5.8 1.01 7.1 1.62 | (a) (a) (a) (a) (a) | 4.5 1.08 1.35 | (a) | 14.7 (a) 4.91 (a) 1.04 6.87 | (d) (d) (d) (d) | ||||||||||||
| Dy Ho | 5.3 | (a) | 7.44 1.34 | (a) | (a) 7.74 | (d) | ||||||||||||
| Er Tm | 0.72 | (a) | 4.55 | (d) | ||||||||||||||
| Yb Lu Hf Ta W ppb Re ppb Os ppb | 4.5 0.64 3.1 0.77 | (a) (a) (a) (a) | 3.7 0.58 3.8 0.44 150 | (a) (a) (a) | (a) 4.32 (a) 0.651 | (d) (d) | ||||||||||||
| Ir ppb Pt ppb | 3.6 | (a) | ||||||||||||||||
| Au ppb Th ppm U ppm | 1.03 0.27 | (e) 1.28 | 0.9 (e) 0.356 (a) | (a) (a) | technique: (a) INAA, (b) OES, (c ) , (d) IDMS, (e) radiation couting, (f) conventional wet, (g) XRF, (h) SSMS |
Table 1b. Chemical composition of 12052.
| reference weight SiO2 % TiO2 Al2O3 FeO MnO MgO CaO Na2O K2O P2O5 S % sum | Taylor | 71 Tatsumoto71 |
|---|---|---|
| Sc ppm V Cr Co Ni Cu Zn Ga Ge ppb As Se Rb | 54 160 3300 42 21 5 | (h) (h) (h) (h) (h) (h) |
| Sr Y Zr Nb Mo Ru Rh Pd ppb Ag ppb Cd ppb In ppb | 110 48 130 6 0.03 | (h) (h) (h) (h) (h) |
| Sn ppb Sb ppb Te ppb | 0.16 | (h) |
| Cs ppm Ba La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Hf Ta W ppb Re ppb Os ppb Ir ppb Pt ppb | 0.03 65 6.3 21 2.6 17 6.3 0.9 9 2 9.6 2.7 7.2 1.1 5.3 | (h) (h) (h) (h) (h) (h) (h) (h) (h) (h) |
| Au ppb Th ppm U ppm technique: | 1.15 0.3 (a) INA | (h) 1.282 1.231 1.411 (d) (h) 0.365 0.347 0.404 (d) AA, (b) OES, (c) , (d) IDMS, (e) ra |
Chromite-ulvöspinel: Gibb et al. (1970) determined the composition of Al-chromite cores and Ti-ulvöspinel rims
Iron: Champness et al. (1971) report about 2 % Ni and 1.5 % Co in metallic iron grains.
Chemistry
Section titled “Chemistry”The major element composition was determined by Maxwell and Wiik (1971), Kushiro et al. (1971) and Compston et al. (1971). Trace elements were determined by Taylor et al. (1971), Wanke et al. (1971) and Schnetzler et al. (1971)(figure 5).
Radiogenic age dating
Section titled “Radiogenic age dating”Murthy et al. (1971) determined a Rb-Sr mineral isochron with age $2.92 \pm 0.18$ b.y. (figure 8) and Compston et al. (1971) determined $3.28 \pm 0.19$ b.y. (figure 7).
Cosmogenic isotopes and exposure ages
Section titled “Cosmogenic isotopes and exposure ages”Burnett et al. (1975) determined an exposure age of $230 \pm 40$ m.y. by $^{81}\text{Kr}/^{83}\text{Kr}$ and Marti and Lugmair (1971) determined $129 \pm 7$ m.y by $^{81}\text{Kr}/^{83}\text{Kr}$ . Hintenberger et al. (1971) also determined exposure ages for 12052 using $^{3}\text{He}$ (120 m.y.), $^{21}\text{Ne}$ (140 m.y.) and $^{38}\text{Ar}$ (130 m.y.).
Other Studies
Section titled “Other Studies”Bogard et al. (1971) and Hintenberger et al. (1971) reported the content and isotopic composition of rare gases in 12052.
Processing
Section titled “Processing”A large amount of 12052 was used for the biopool sample.
List of Photo #s for 12052
Section titled “List of Photo #s for 12052”| 2350 01 1 11000 115 101 | |
|---|---|
| S69-62806 - 62819 | |
| S69-63832 - 63834 | |
| S70-44847 - 44848 | 12052,1 |
| S70-44630 - 44639 | 12052,2 |
| S70-49562 - 49563 | TS color |
| S70-49835 - 49836 | TS color |
| S70-17972 - 17973 | TS color |
| S75-34165 - 34170 | mug color |
| S75-34269 | sawing |
| S75-34255 - 34257 | saw cut |
technique: (a) INAA, (b) OES, (c), (d) IDMS, (e) radiation couting, (f) conventional wet, (g) XRF, (h) SSMS

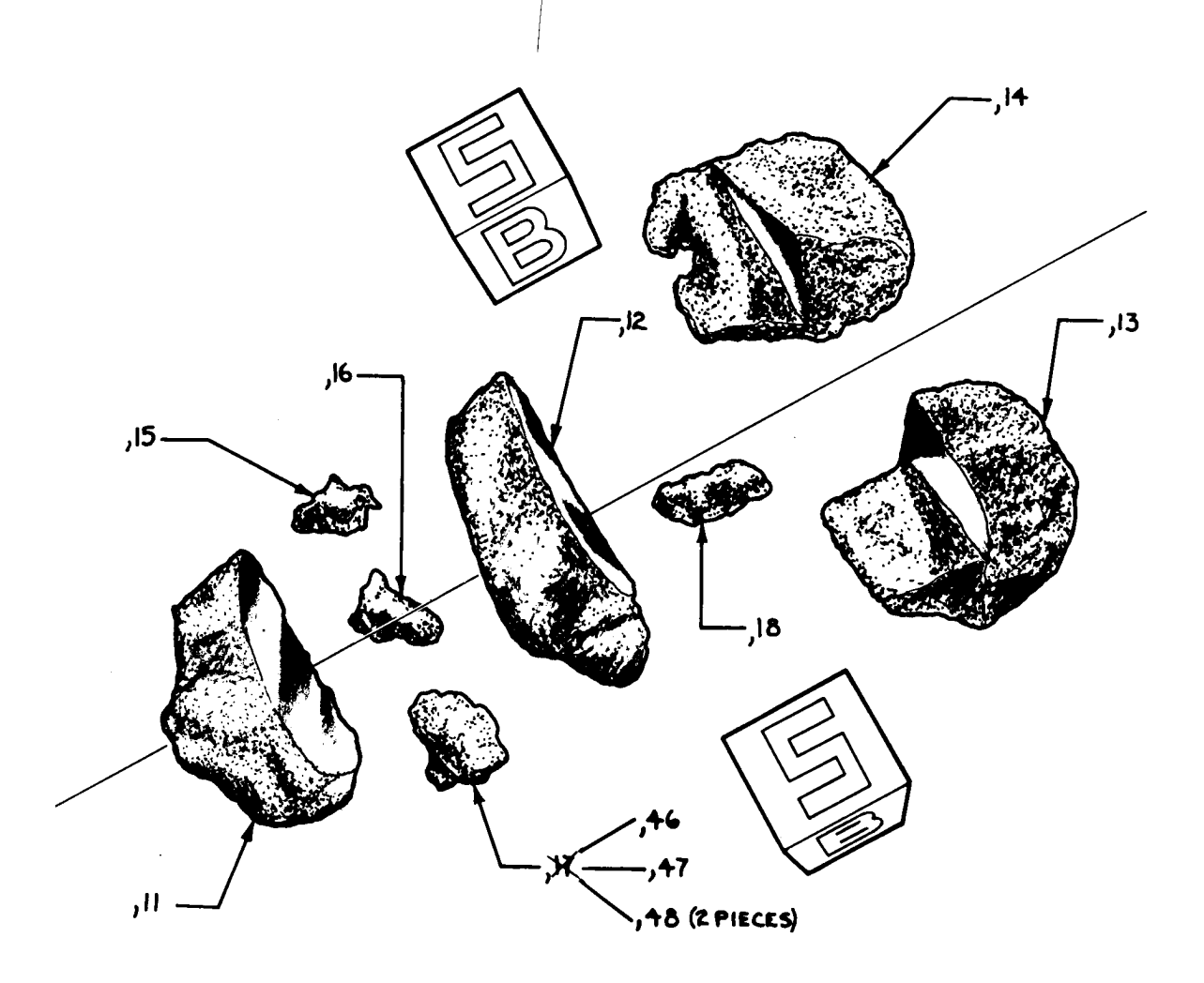
Figure 9: Photo of broken surface of 12052,1 showing pyroxene crystals in vugs. NASA #S70-44847. Scale 1 cm.
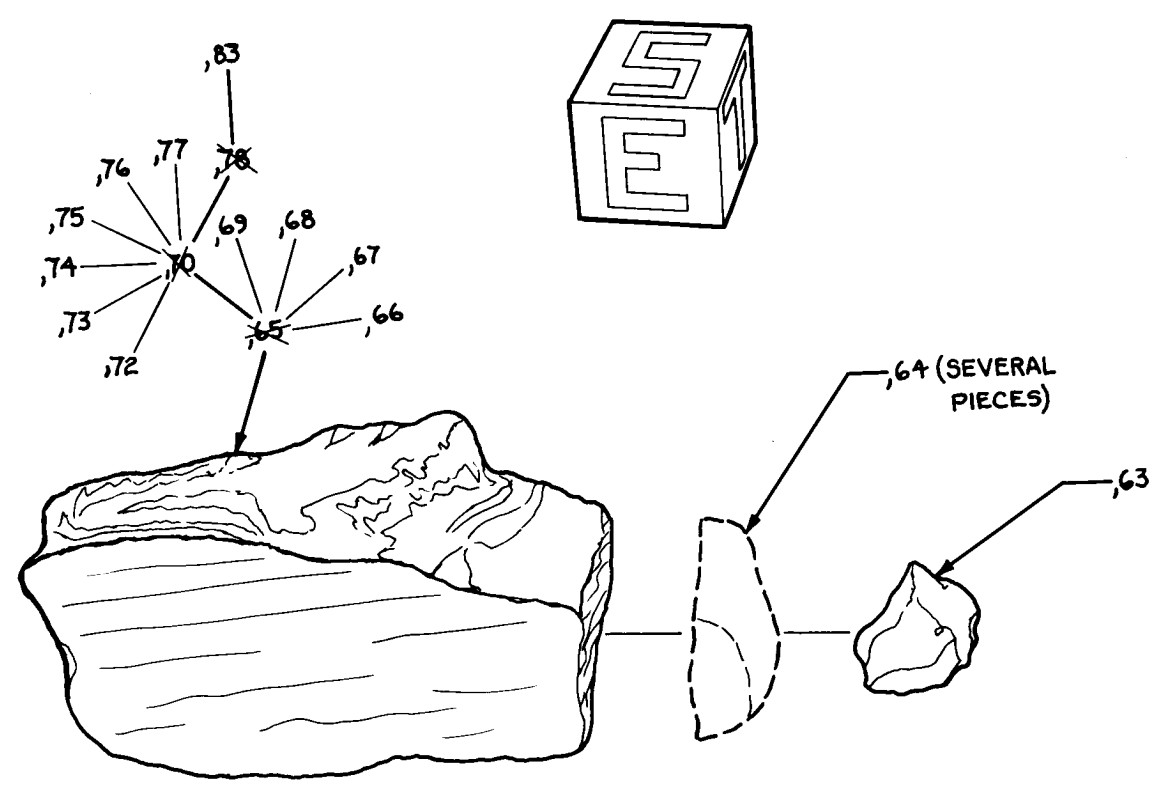
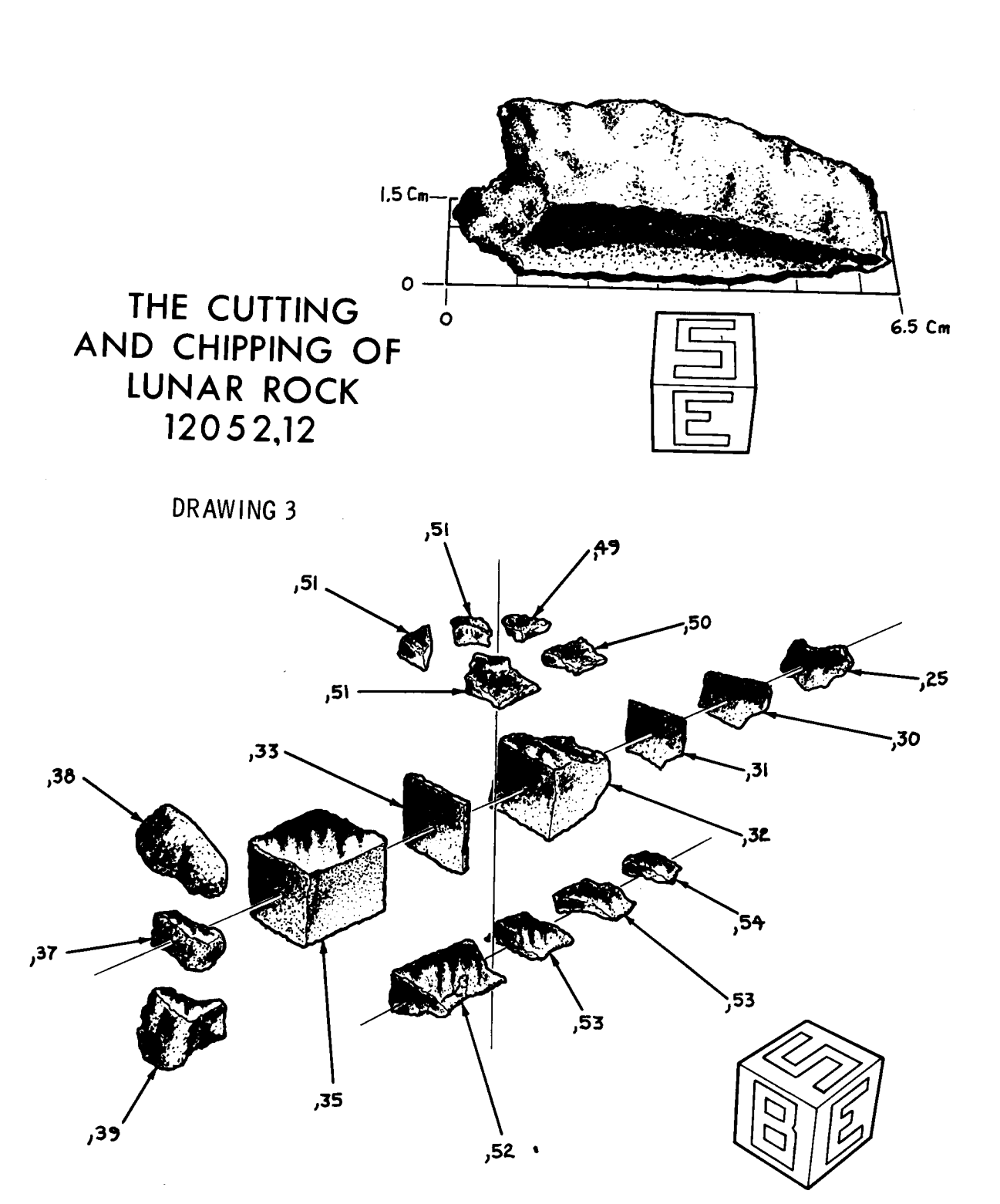
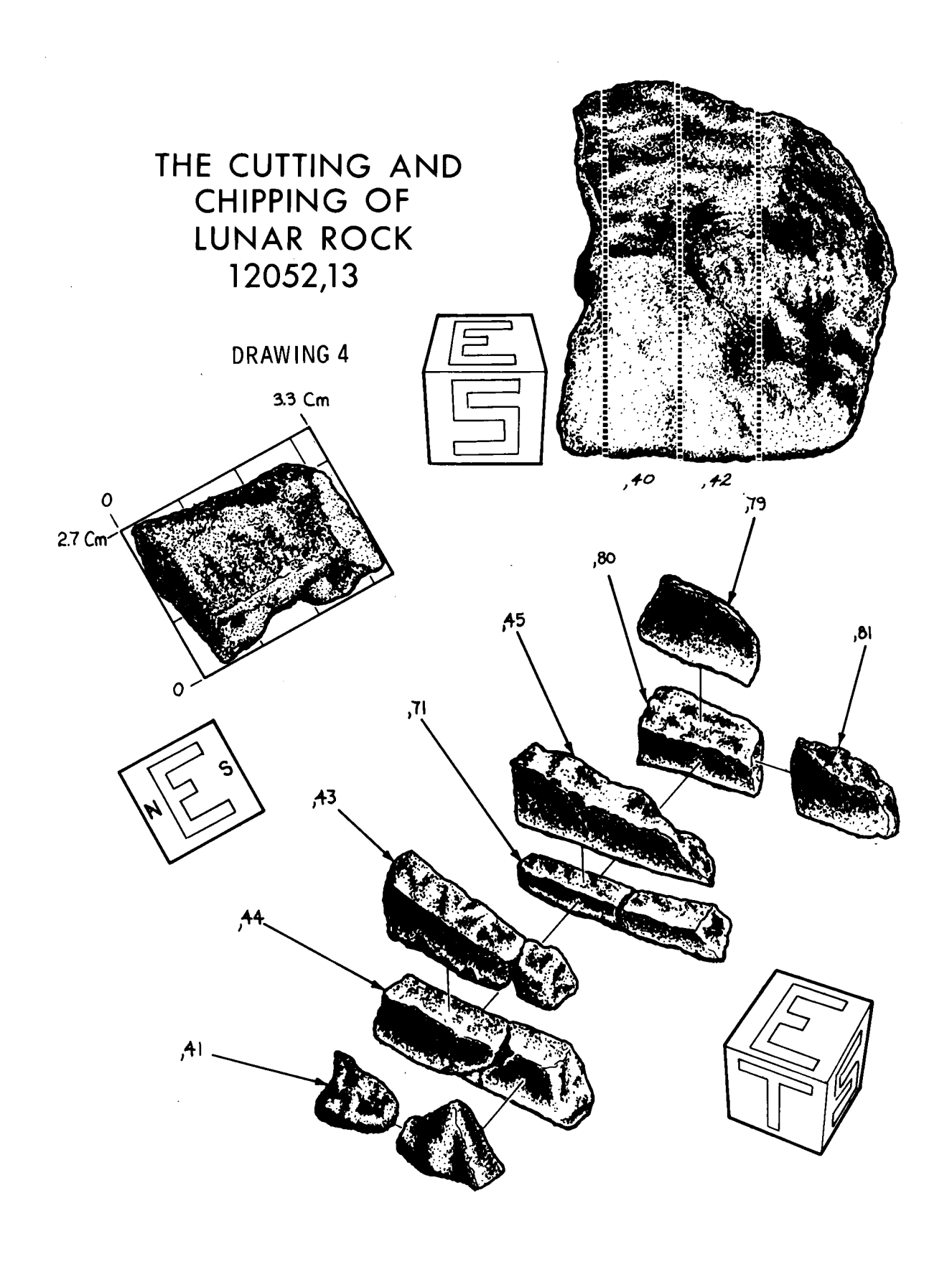
References for 12052
Section titled “References for 12052”Baldridge W.S., Beaty D.W., Hill S.M.R. and Albee A.L. (1979) The petrology of the Apollo 12 pigeonite basalt suite. Proc. 10th Lunar Planet. Sci. Conf. 141-179.
Bence A.E., Papike J.J. and Prewitt C.T. (1970) Apollo 12 clinopyroxene chemical trends. Earth Planet. Sci. Lett. 8, 393-399.
Bence A.E., Papike J.J. and Lindsley D.H. (1971) Crystallization histories of clinopyroxenes in two porphyritic rocks from Oceanus Procellarum. Proc. 2nd Lunar Sci. Conf. 559-574.
Bence A.E. and Papike J.J. (1972) Pyroxenes as recorders of lunar basalt petrogenesis: Chemical trends due to crystalliquid interaction. Proc. 3rd Lunar Sci. Conf. 431-469.
Bence A.E. and Autier B. (1972) Secondary ion analysis of pyroxenes from two porphritic lunar basalts. In The Apollo 15 Lunar Samples, 191-194. The Lunar Science Institute, Houston.
Bogard D.D., Funkhouser J.G., Schaeffer O.A. and Zahringer J. (1971) Noble gas abundances in lunar material-cosmic ray spallation products and radiation ages from the Sea of Tranquility and the Ocean of Storms. J. Geophys. Res. 76, 2757-2779.
Compston W., Berry H., Vernon M.J., Chappell B.W. and Kay M.J. (1971) Rubidium-strontium chronology and chemistry of lunar material from the Ocean of Storms. Proc. 2nd Lunar Sci. Conf. 1471-1485.
Gibb F.G.F., Stumpfl E.F. and Zussman J. (1970) Opaque minerals in an Apollo 12 rock. Earth Planet. Sci. Lett. 9, 217-224.
Gibb F.G.F. and Zussman J. (1971) Zoned olivine in four Apollo 12 samples. Earth Planet. Sci. Lett. 11, 161-167.
Hintenberger H., Weber H.W. and Takaoka N. (1971) Concentrations and isotopic abundances of the rare gases in lunar matter. Proc. 2nd Lunar Sci. Conf. 1607-1625.
Kushiro I., Nakamura Y., Kitayama K. and Akimoto S-I. (1971) Petrology of some Apollo 12 crystalline rocks. Proc. 2nd Lunar Sci. Conf. 481-495.
Marti K. and Lugmair G.W. (1971) Kr81-Kr and Kr-Ar40 ages, cosmic-ray spallation products and neutron effects in lunar samples from Oceanus Procellarum. Proc. 2nd Lunar Sci. Conf. 1591-1605.
Maxwell J.A. and Wiik H.B. (1971) Chemical composition of Apollo 12 lunar samples 12004, 12033, 12051, 12052 and 12065. Earth Planet. Sci. Lett. 10, 285-288.
Murthy V.R., Evensen N.M., Jahn B.-M. and Coscio M.R. (1971) Rb-Sr ages and elemental abundances of K, Rb, Sr and Ba in samples from the Ocean of Storms. Geochim. Cosmochim. Acta 35, 1139-1153.
Neal C.R., Hacker M.D., Snyder G.A., Taylor L.A., Liu Y. G. and Schmitt R.A. (1994a) Basalt generation at the Apollo 12 site, Part 1: New data, classification and re-evaluation. Meteoritics 29, 334-348.
Neal C.R., Hacker M.D., Snyder G.A., Taylor L.A., Liu Y. G. and Schmitt R.A. (1994b) Basalt generation at the Apollo 12 site, Part 2: Source heterogeneity, multiple melts and crustal contamination. Meteoritics 29, 349-361.
Nyquist L.E., Bansal B.M., Wooden J. and Wiesmann H. (1977) Sr-isotopic constraints on the peterogenesis of Apollo 12 mare basalts. Proc. 8th Lunar Sci. Conf. 1383-1415.
O’Kelley G.D., Eldridge J.S., Schonfeld E. and Bell P.R. (1971a) Abundances of the primordial radionuclides K, Th, and U in Apollo 12 luanr samples by nondestructive gammaray spectroscopy: implications for the origin of lunar soils. Proc. Second Lunar Sci. Conf. 1159-1168.
O’Kelley G.D., Eldridge J.S., Schonfeld E. and Bell P.R. (1971b) Cosmogenic radionuclide concentrations and exposure ages of lunar samples from Apollo 12. Proc. Second Lunar Sci. Conf. 1747-1755.
Papike J.J., Bence A.E., Brown G.E., Prewitt C.T. and Wu C.H. (1971) Apollo 12 clinopyroxenes: Exsolution and epitaxy. Earth Planet. Sci. Lett. 10, 307-315.
Papike J.J., Hodges F.N., Bence A.E., Cameron M. and Rhodes J.M. (1976) Mare basalts: Crystal chemistry, mineralogy and petrology. Rev. Geophys. Space Phys. 14, 475-540.
Schnetzler C.C. and Philpotts J.A. (1971) Alkali, alkaline earth, and rare earth element concentrations in some Apollo 12 soils, rocks, and separated phases. Proc. 2nd Lunar Sci. Conf. 1101-1122.
Takeda H. (1972) Structural studies of rim augite and core pigeonite from lunar rock 12052. Earth Planet. Sci. Lett. 15, 65-71.
Tatsumoto M., Knight R.J. and Doe B.R. (1971) U-Th-Pb systematic of Apollo lunar samples. Proc. 2nd Lunar Sci. Conf. 1521-1546.
Taylor S.R., Rudowski R., Muir P., Graham A. and Kaye M. (1971) Trace element chemistry of lunar samples from the Ocean of Storms. Proc. 2nd Lunar Sci. Conf. 1083-1099.
Wänke H., Wlotzka F., M. and Rieder R. (1971) Apollo 12 samples: Chemical composition and its relation to sample locations and exposure ages, the two component origin of the various soil samples and studies on lunar metallic particles. Proc. 2nd Lunar Sci. Conf. 1187-1208.