Sample 15028
15027 – 51 grams 15028 – 59.4 grams Regolith Breccia
Section titled “15027 – 51 grams 15028 – 59.4 grams Regolith Breccia”

Figure 3: Surface photo of 15027 and 15028. Figure 4: Location of 15027 on map of Apollo 15. AS15-86-11606


Figure 1: Photo of 15027. S71-43635 Figure 2 a,b: Photos of 15028. Cube is 1 inch. S71- 43639 and 43641
Introduction
Section titled “Introduction”15027 and 15028 are both glass-coated breccias found together near the LM. They were returned in the same bag (DB162), but are identified as independent objects on lunar surface (figure 3).
Petrography
Section titled “Petrography”McKay et al. (1989) reported that the maturity index for 15028 was Is /FeO = 26.
Kridelbaugh et al. (1972) reported that about 30 % of 15028 is glass and that it had numerous clasts of KREEP basalt. Uhlmann et al. (1981) used the glass to estimate cooling rate (fast).
Chemistry
Section titled “Chemistry”Wanke et al. (1976) found 15027 and 15028 to have similar composition. As is the case for other Apollo 15 breccia samples, 15027 and 15028 have higher trace element content than Apollo 15 soils (figure ).
Other Studies
Section titled “Other Studies”Bogard determined the rare gas content and isotopic ratio for 15028 – reported in McKay et al. (1989).
References for 15028
Section titled “References for 15028”Butler P. (1971) Lunar Sample Catalog, Apollo 15. Curators’ Office, MSC 03209
Kridelbaugh S.J., Grieve RAF and Weill D.F. (1972) Glass compositions in breccias 15028 and 15059. In The Apollo 15 Lunar Samples, 123-125.
LSPET (1972a) The Apollo 15 lunar samples: A preliminary description. Science 175, 363-375.
LSPET (1972b) Preliminary examination of lunar samples. Apollo 15 Preliminary Science Report. NASA SP-289, 6 1—6-28.
McKay D.S., Morris R.V. and Wentworth S.J. (1984) Maturity of regolith breccias as revealed by ferromagnetic and petrographic indicies (abs). Lunar Planet. Sci. XV, 530 531. Lunar Planetary Institute, Houston.
McKay D.S., Bogard D.D., Morris R.V., Korotev R.L., Wentworth S.J. and Johnson P. (1989) Apollo 15 regolith breccias: Window to a KREEP regolith. Proc. 19th Lunar Sci. Conf. 19-41. Lunar Planetary Institute, Houston.
Ryder G. (1985) Catalog of Apollo 15 Rocks (three volumes). Curatoial Branch Pub. # 72, JSC#20787
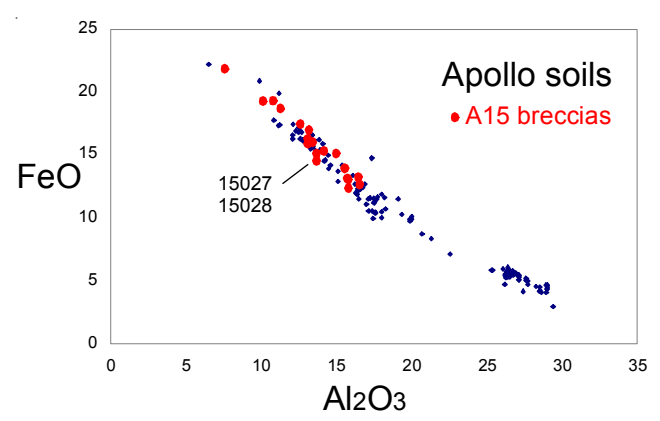
Figure 5: Composition of Apollo soils, Apollo 15 breccias and 15028.
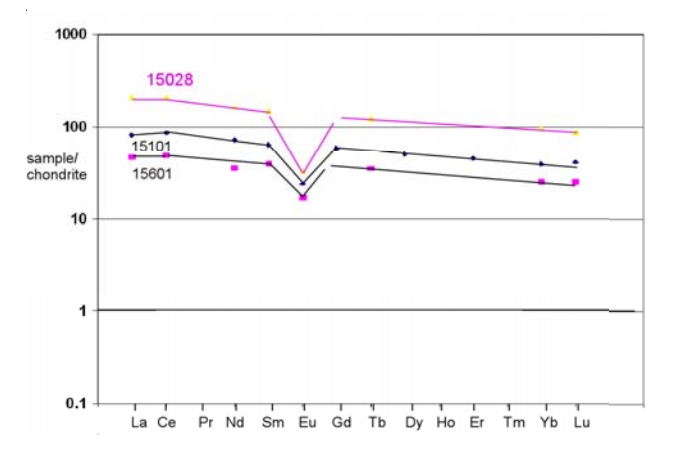
Figure 6: Thace element composition of 15028 and some soils.
Swann G.A., Hait M.H., Schaber G.C., Freeman V.L., Ulrich G.E., Wolfe E.W., Reed V.S. and Sutton R.L. (1971b) Preliminary description of Apollo 15 sample environments. U.S.G.S. Interagency report: 36. pp219 with maps
Swann G.A., Bailey N.G., Batson R.M., Freeman V.L., Hait M.H., Head J.W., Holt H.E., Howard K.A., Irwin J.B., Larson K.B., Muehlberger W.R., Reed V.S., Rennilson J.J., Schaber G.G., Scott D.R., Silver L.T., Sutton R.L., Ulrich G.E., Wilshire H.G. and Wolfe E.W. (1972) 5. Preliminary Geologic Investigation of the Apollo 15 landing site. In Apollo 15 Preliminary Science Rpt. NASA SP-289. pages 5-1-112.
Uhlmann D.R., Yinnon H. and Fang C.-Y. (1981) Simplified model evaluation of cooling rates for glass-containing lunar compositions. Proc. 12th Lunar Planet. Sci. Conf. 281-288.
Wänke H., Baddenhausen H., Blum K., Cendales M., Dreibus G., Hofmeister H., Kruse H., Jagoutz E., Palme C., Spettel B., Thacker R. and Vilcsek E. (1977a) On the chemistry of lunar samples and achondrites. Primary matter in the lunar highlands: A re-evaluation. Proc. 8th Lunar Sci. Conf. 2191-2213.
Table 1. Chemical composition of 15028
| reference McKay89 | 15028 15027 Wanke77 | Kriedelbaugh72 | Uhlmann81 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| weight SiO2 % TiO2 Al2O3 FeO MnO | 2 13.6 14.5 0.19 | 48.9 (a) 1.79 (a) 12.88 (a) 14.2 (a) 0.2 | 49.4 1.89 13.78 14.2 0.2 | (b) | glass (b) 48 (b) 1.75 (b) 14.7 (b) 14.1 | vein 46.5 1.6 16.5 13.7 | 49 1.4 12.9 14.1 | (c ) (c ) (c ) (c ) | |||
| MgO CaO Na2O K2O P2O5 S % sum | 9.2 9.8 0.55 | (a) 9.25 (a) 10.4 (a) 0.58 0.41 0.36 | 9.2 10.44 0.6 0.42 0.394 | (b) 8.7 (b) 10.3 (b) 0.59 (b) 0.41 (b) 0.3 | 8.6 10.7 0.64 0.36 0.37 | 7.4 9.5 0.6 0.4 | (c ) (c ) (c ) (c ) (c ) | ||||
| Sc ppm V Cr Co Ni Cu Zn Ga Ge ppb As Se | 28.7 83 2410 35.2 135 | (a) 39 | (a) 29.9 (a) 95.6 (a) 2570 (a) 200 5.3 8 3.36 300 63 350 | 30.8 98 2620 39 180 | (b) (b) (b) (b) (b) (b) (b) (b) (b) (b) | (b) 1600 | 950 | 1300 | (c ) | ||
| Rb Sr Y Zr Nb Mo Ru Rh | 170 660 | 10.7 (a) 139 154 (a) 666 48 | 145 158 662 47 | (b) (b) (b) (b) (b) | C Meyer 15027 2011 51 grams | ||||||
| Pd ppb Ag ppb Cd ppb In ppb Sn ppb Sb ppb Te ppb | ,2 ,1 ,3 ,0 1g 1g PB 49 g ,6 ,7 | ||||||||||
| Cs ppm Ba La Ce Pr | 0.44 523 48.6 127 | (a) 0.53 (a) 501 (a) 46.9 (a) 130 16.7 | 515 47.3 129 | (b) (b) (b) (b) (b) | TS | ||||||
| Nd Sm Eu Gd Tb Dy Ho Er Tm | 73 21.9 1.86 4.42 | (a) 74 | (a) 19.7 (a) 1.77 26.2 (a) 4.53 26.9 5.6 17.4 | 75 (b) 20.7 (b) 1.81 (b) (b) 4.54 (b) 26.4 (b) (b) (b) | C Meyer 15028 2011 59.4 grams ,8 ,9 ,7 ,0 ,1 1g 0.5 g | ||||||
| Yb Lu Hf Ta W ppb Re ppb Os ppb | 15.5 2.12 18 2.08 | (a) 17 | (a) 15.8 (a) 2.18 (a) 2.01 980 0.51 | 15.7 2.17 17 2.05 | (b) (b) (b) (b) (b) (b) | 0.8 g PB 56.6 g ,2 ,6 | |||||
| Ir ppb Pt ppb | 3.8 | (a) | 3 | (b) | TS | ||||||
| Au ppb Th ppm U ppm technique: (a) INAA, (b) various, (c ) elec. Probe | 9.6 8.3 2.37 | (a) 4 | (a) 7.49 (a) 2.37 | 7.45 2.3 | (b) (b) (b) |